Benzopyrene
There are multiple types of Benzopyrene. The first and most common is Benzo[a]pyrene and the others and significantly less common types include Benzo[e]pyrene and Benzo[d]pyrene. Benzo[a]pyrene is a carcinogen. It is the major cancer causing agent in cigarettes.
Benzo[a]pyrene
Chemical Formula: C20 H12
IUPAC Name: 3,4- Benzapyrene
Classical System Name: Benzopyrene
These are both structural diagrams of Benzo[a]pyrene.
pyrene-3D-balls.png)
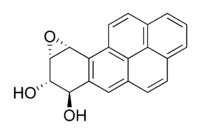
This is an example of how Benzo[a]pyrene breaks the bonds that hold DNA
together and disrupt the genes for mitosis causing a cell to become cancerous.
Chemical Family:
Polycyclic Aromatic Hydrocarbons:
Polycyclic aromatic hydrocarbons which are also referred to as polyarenes, are formed as products of incomplete combustion of fossil fuels as well as other organic matter. Compounds which are polycyclic aromatic hydrocarbons are mostly all carcinogens.
The functional group of polycyclic aromatic hydrocarbons is a benzene ring or multiple benzene rings. Benzo[a]pyrene has 5 benzene rings therefore it is an polycyclic aromatic hydrocarbon.
Physical and Chemical Properties of Benzo[a]pyrene:
Physical Properties:
1. Melting Point: 177°C -180°C
2.Boiling Point: 495°C
3. Yellow crystals or powder.
4. Found in cigarette smoke.
Chemical Properties:
1. It is a stable compound in that it is incompatible with strong oxidizing agents.
2. Insoluble in water.
3.It undergoes photo-oxidation after irradiation in indoor sunlight or by fluorescent light in organic solvents.
4. In liquid form it is a threat to the environment as it can easily get into the soil and contaminate ground water or nearby waterways.. Immediate steps should be taken to limit its spread to the environment.
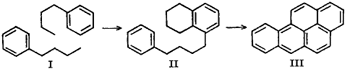
Methods of Synthesis:
Benzo[a]pyrene is synthesized during incomplete combustion. This may occur when a cigarette is being smoked or in substances that are in the cigarette to begin with such as tar or other organic compounds. It is created when two simpler hydrocarbons react such as C6 and C4 hydrocarbons which is shown by the diagram below.